
News


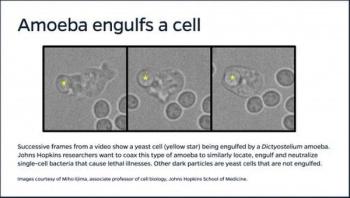
Drawing on their expertise in control systems and cell biology, Johns Hopkins University researchers are setting out to design and test troops of self-directed microscopic warriors that can locate and neutralize dangerous strains of bacteria.


Dr. Margaret Chan, director-general of the World Organization (WHO), issues a statement on preparing for the long haul of Zika readiness.

It’s been one year since the World Health Organization (WHO) declared Zika a public health emergency. The virus, transmitted through the Aedes aegypti mosquito, has since been declared to be a long-term problem rather than an emergency, but Zika continues to concern health professionals. Professors in Notre Dame’s Department of Biological Sciences and members of the Eck Institute reflect on the outbreak, the challenges presented by the virus and the work yet to be done to help health professionals and key decision makers protect their citizens.

With a $416,000 grant from the National Institutes of Health (NIH), SLU scientists will continue work to cure hepatitis B, building on significant findings published in two recent papers. John Tavis, PhD, professor of molecular microbiology and immunology at Saint Louis University, aims to advance our understanding of how the hepatitis B drug replicates in order to develop a new drug that, in combination with other medications, could cure the viral infection.
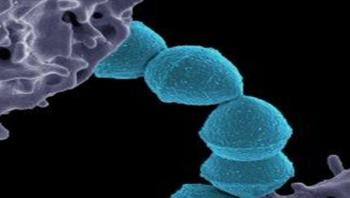
Beginning in the mid-1980s, an epidemic of severe invasive infections caused by Streptococcus pyogenes (S. pyogenes), also known as group A streptococcus (GAS), occurred in the United States, Europe, and elsewhere. The general public became much more aware of these serious and sometimes fatal infections, commonly known as the “flesh-eating disease.” Potent cytotoxins produced by this human pathogen contribute to the infection. A new study in The American Journal of Pathology reports that the bacteria’s full virulence is dependent on the presence of two specific cytotoxins, NADase (SPN) and streptolysin O (SLO).

A Virginia Tech Carilion expert in infectious diseases provides context following a series of media reports noting that this year’s influenza season has reached “epidemic” levels.

Perena Gouma, a professor in the Materials Science and Engineering Department at The University of Texas at Arlington, has published an article in the journal Sensors that describes her invention of a hand-held breath monitor that can potentially detect the flu virus.
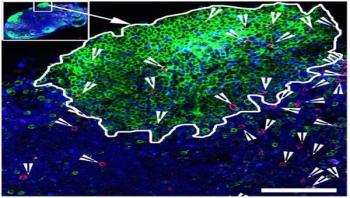
When someone is HIV-positive and takes antiretroviral drugs, the virus persists in a reservoir of infected cells. Those cells hide out in germinal centers, specialized areas of lymph nodes, which most "killer" antiviral T cells don't have access to.











In a two-day conference organized in collaboration between the Maltese Presidency of the Council of the European Union and European Centre for Disease Prevention and Control (ECDC), HIV experts from across the European Union discussed how to reverse this trend and how to prepare Europe to achieve the set target of ending AIDS by 2030.

Using immature stem cells to create a miniature model of the gut in the laboratory, researchers at Washington University School of Medicine in St. Louis and the University of Pittsburgh have determined how infection-causing enteroviruses enter the intestine.


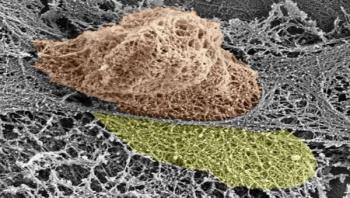
One of the mysteries of the living body is the movement of cells – not just in the blood, but through cellular and other barriers. New research at the Weizmann Institute of Science has shed light on the subject, especially on the movement of immune cells that race to the sites of infection and inflammation. The study revealed that these cells – white blood cells – actively open large gaps in the internal lining of the blood vessels, so they can exit through the vessel walls and rapidly get to areas of infection.
