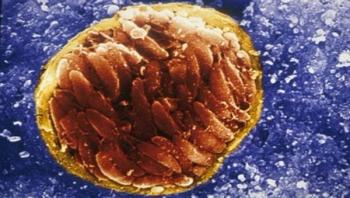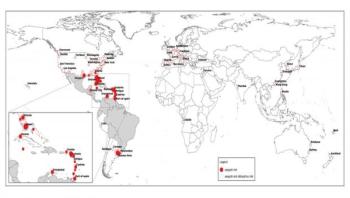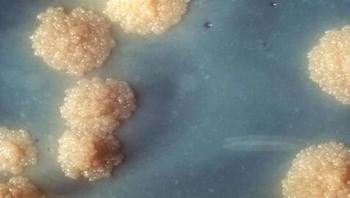
Although tuberculosis (TB) is commonly thought of as being a disease that mainly affects nineteenth century poets and Victor Hugo characters, it is still the second-most common cause of mortality from an infectious disease in the world, killing nearly three people every minute. Every March 24, on World TB Day, the global health community recognizes the work of Robert Koch, who announced on that date in 1882 his discovery of Mycobacterium tuberculosis, the bacteria that causes TB.