Infection Control Today® Editorial Staff
Articles by Infection Control Today® Editorial Staff

Whether it is a birthday, an anniversary, or no reason at all, infection prevention personnel love to give and receive gifts that help at the end of a stressful day. Infection Control Today® offers gift ideas for prevention personnel and their families.
William Shutze, MD, FACS, discusses how infection prevention impacts vascular health, emphasizing key strategies and the role of vascular surgeons in maintaining vascular well-being.

Infection Control Today's Product Locator is a monthly column highlighting some of the latest advanced technology in infection prevention.

Chaitenya Razdan, CEO and founder of Care+Wear, discusses the development of adaptive clothing for patients with PICC lines and NICU infants, focusing on comfort, dignity, and infection prevention.
Nature Communications published the results of a study on ARCT-154, CSL’s sa-mRNA COVID-19 vaccine. The study showed that it is well-tolerated, immunogenic, and effective against multiple strains.

Happy Memorial Day from Infection Control Today!
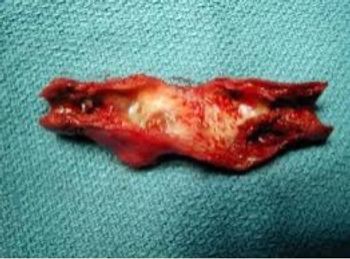
Hadassah Medical Organization's pooled saliva testing for newborns effectively screens for congenital cytomegalovirus, identifying asymptomatic cases and potentially reducing severe cognitive and auditory problems globally.
A recent study published in JAMA discusses the efficacy and safety of acetaminophen in sepsis patients.

Discover how SpaceWalker's innovative cooling solutions reduce carbon footprints and ensure pharmaceutical integrity, setting new standards in sustainable cold chain logistics.

Staying Ahead with Company Updates and Product Innovations. Read the news from Crothall Healthcare and Ascendco Health, Freestyle Partners, and Shionogi & Co.
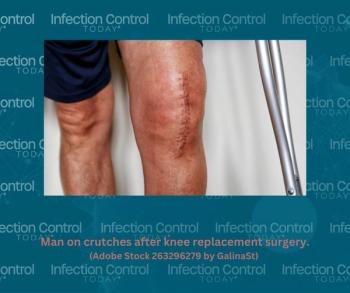
Systematic review identifies a 3.0% prevalence of surgical site infections in knee surgery patients, with factors like diabetes and tobacco contributing. Tailored interventions are crucial.

FDA provides clarity on medical device remanufacturing vs. servicing in new guidance. Ensures industry consistency and compliance with federal regulations for improved device safety and effectiveness.

Happy Hand Hygiene Day! Bradley's 2024 survey unveils American handwashing trends: It increased during illness and travel but decreased after restroom use. Parents are key in fostering hygiene habits.

Staying Ahead with Company Updates and Product Innovations. Read the news from PerfectClean, the FDA, and the CDC.

The FDA has accepted GSK's BLA for a 5-in-1 meningococcal vaccine candidate, which promises streamlined immunization and a potential reduction in invasive meningococcal disease (IMD).

Learn about the FDA's approval of Zevtera (ceftobiprole medocaril sodium) for Staphylococcus aureus infections in adults and pediatric patients, including common side effects and precautions.

Important Recall Notice: AvKARE recalls Atovaquone Oral Suspension due to potential Bacillus cereus contamination. Learn about risks, recommendations, and how to proceed if you have this product.

Explore Agilent Technologies' role in advancing infectious disease research through cutting-edge solutions, from in vitro transcribed RNA vaccine testing to biofilm evaluation.

The CDC has sounded the alarm on a sharp rise in invasive meningococcal disease cases, notably linked to Neisseria meningitidis serogroup Y. Health care providers are urged to stay vigilant.
In the ongoing fight against COVID-19, the FDA has granted Emergency Use Authorization for Pemgarda, a COVID-19 infusion drug. Read on for more.

CenTrak, the industry leader in real-time location technology, introduces the groundbreaking BLE Multi-Mode Platform, setting a new standard for Real-Time Location Systems (RTLS) in health care. Discover how this innovative solution enhances location data precision and streamlines operations for health care organizations.

Jai Shah, CEO of Kahuna Workforce Solutions, discusses nursing challenges from staffing shortages to burnout. Learn how digitized competency management transforms patient care, patient safety, and nurse satisfaction.

Meet the experts shaping infection prevention: Infection Control Today's Editorial Board members share insights, experiences, and cutting-edge strategies to enhance health care safety and quality. Meet Tabrikah Mohammed, MSc, BSN, CIC, CPHQ, FAPIC.

Pfizer Inc, has released promising top-line data for its Abrysvo vaccine, demonstrating high efficacy against respiratory syncytial virus (RSV) in adults aged 60 and older. These findings from the Phase 3 clinical trial hold significant implications for infection control personnel working to combat RSV infections, especially in vulnerable populations.

Health care workers urged to review biosecurity as USDA confirms first HPAI case since 2022 in domestic birds during ongoing outbreak.
Syphilis cases surge, sparking concerns. Deborah Sesok-Pizzini, MD, discusses what infection prevention and control personnel should know about testing evolution, prevention strategies, and innovations combating spread.

Learn about FendX Technologies' breakthrough nanotechnology, including REPELWRAP film and a spray formulation, designed to prevent the spread of infectious diseases on various surfaces.

Tina Platania discusses the significance of continuous glucose monitors (CGM) in diabetes care, highlighting CCS's individualized approach based on medical necessity. From carefully considering patient needs to collaborating with providers and payers, CCS ensures a highly personalized treatment plan, reflecting their commitment to improving overall health.

CSL achieves a global milestone as Japan approves ARCT-154, the first sa-mRNA COVID-19 vaccine, showcasing CSL's commitment to innovation, efficacy, and prolonged protection against variants. Jonathan Edelman, MD, senior VP of CSL Vaccine’s Innovation Unit, speaks with ICT.

FDA guides the transition from emergency-use-labeled Paxlovid to approved labeling, impacting dispensing sites, providers, and patients.




















