
News
Advertisement


Advertisement




Advertisement





















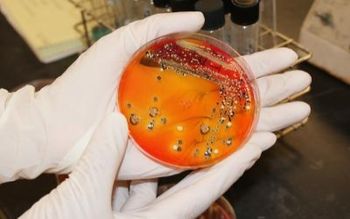
Earlier this year, an outbreak of salmonella caused by infected eggs resulted in thousands of illnesses before a costly recall could be implemented. Now, University of Missouri researchers have created a new test for salmonella in poultry and eggs that will produce faster and more accurate results than most currently available tests. The new test could have prevented the contaminated eggs from being shipped to stores.

Advertisement
Advertisement
Trending on Infection Control Today
1
Bug of the Month: The Quiet Guest in the Dust
2
What Is Effective Preparedness for Emerging Respiratory Viruses? Shazia Irum, MSC, MBA, RN, CIC, CPHQ, FAPIC, answers
3
Continuous Photohydrolysis Disinfection Cuts MDROs, COVID-19, and Hospital Transfers in Long-Term Care, Study Finds
4
Influenza D and Canine Coronavirus: Why Underrecognized Animal Viruses May Be the Next Respiratory Threat
5