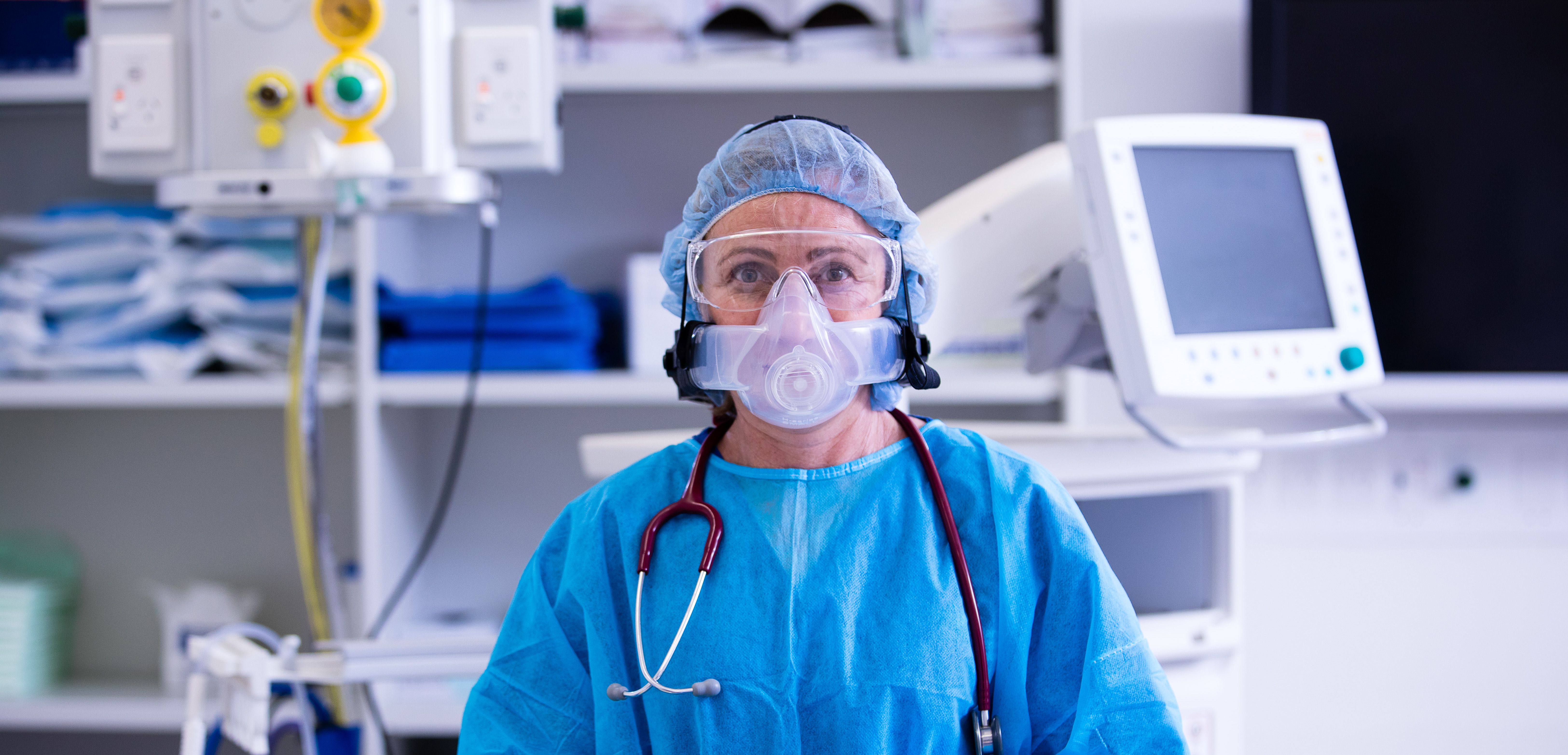
Personal Protective Equipment
Latest News



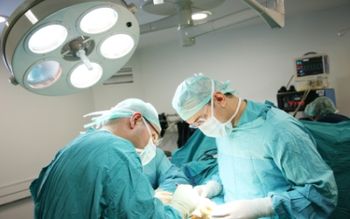





How over-the-head isolation gowns increase staff and visitor safety.

Material scientists at the University of Manchester, working in collaboration with universities in China, have created a 'durable and washable, concrete-like' composite material made from antibacterial copper nanoparticles.




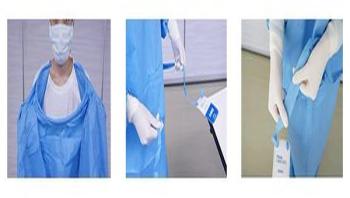
In the healthcare setting, there is an increasing need for a self-donning surgical gown that healthcare personnel can don without the need for any assistance. Also, in the context of crisis management for the Ebola virus and other severe infectious diseases, use of a gown that can be donned and removed quickly and safely as infection protection to prevent transmission to the environment is more important than ever.

The quality and vitality of the operating room is often a balance between managing patients known or suspected with infectious disease and managing potential staff occupational exposure risks associated with treating patients. With exposure risks to emerging and re-emerging microorganisms at an unparalleled high, measuring, analyzing, and preventing exposures among surgical staff is more important now than ever.


A University of Alberta engineering researcher has developed a new way to treat common surgical masks so they are capable of trapping and killing airborne viruses. His research findings appear in the prestigious journal Scientific Reports, published by Nature Publishing Group.

As personal protective equipment (PPE) continues to play an integral role in prevention of transmission of infection in the healthcare setting, we discover by looking back at the history of protection of healthcare workers (HCWs) and prevention of spread of infection, that the concept is several centuries old.
According to 2014 occupational incident surveillance data from nearly 30 U.S. hospitals, when an employee experiences a splash or splatter of blood or body fluid (e.g. blood or bloody urine) into the eye they are only wearing eye-appropriate personal protective equipment (PPE) 3.5 percent of the time. These mucotaneous exposures are extremely high risk. Just as the eyes are the windows to the soul, they are the frontlines for risk of disease transmission from patient to worker. If we experience fatigue, allergies, irritation, or infection our eyes become even more susceptible to microorganisms that come into contact with them.