
The electrostatic sprayer method kills nearly 100% of pathogens. It also kills the COVID-19 virus. But is that overkill?


The electrostatic sprayer method kills nearly 100% of pathogens. It also kills the COVID-19 virus. But is that overkill?
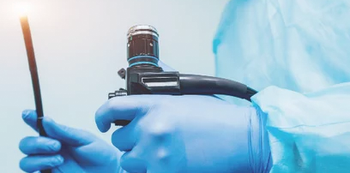
La’Titia Houston MPH, BSN, RN, CIC: “We work not only with the bedside nurses and the sterile processors, but even with our clinicians, our physicians. They want a timeout before the procedure is even performed because they want to ensure that the scope did pass during the high-level disinfection procedure.”
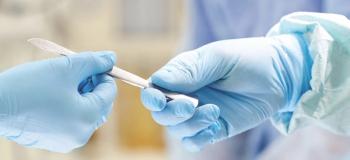
No health care worker is immune from the dangers of handling sharps. Physicians hold a rate just under that of nurses, mostly related to use of scalpels, but are less likely to report these injuries.

Adenosine triphosphate (ATP) bioluminescence needs technological enhancement if it’s to reach its full potential as a disinfection tool, says a study.
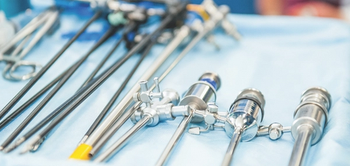
Crystal Heishman, MBA, MSN, RN, ONC, CIC: “You don’t ever want to go into a sterilization department and say, ‘You’re doing this wrong’. Because they’re the subject matter experts. You want to learn. You want to learn the process. You want to work together because it makes a stronger partnership.”

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending October 22.
Brian Flannigan: “The reason why water quality and water safety is so important in sterile processing is that there have been direct connections made between the water systems and hospital infections: operating room infections, asset life problems, maintenance problems, staining and discoloration of equipment.”

The International Association of Healthcare Central Service Materiel Management (IAHCSMM) has joined forces with the Association of Surgical Technologists in hiring a federal lobbying firm, McAllister & Quinn, to help enact hazard pay for sterile processors, surgical technologists and surgical assistants for their work during COVID-19 in 2020 and 2021.
IAHCSMM’s Damien Berg: “COVID-19 put a light on sterile processing professionals in a positive way. We became a force multiplier in the hospital by the things we did. And we got known.”

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending July 23.
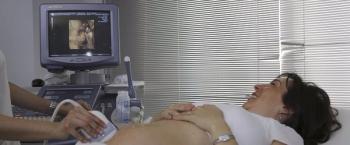
The high-level disinfection process of an ultrasound probe, when indicated, includes documentation that demonstrates high-level disinfection was performed, and patient identifiers were documented to link the ultrasound probe to the patient. This is often referred to as traceability.

Non-ventilator-hospital associated pneumonia prevention is quickly becoming the hot topic among infection preventionists.
Infection preventionists are integral members of the extended sterile processing department team and can be among the department’s biggest supporters.

Holly Taylor, MPH, CIC: Using retired IPs can “create a little bit more bandwidth within the department when you have potentially prolonged vacancies because we do know that IP staffing vacancies last longer than other health care vacancies.”
The infection prevention department at Ascension Texas managed to shore up IP ranks by calling a couple of IPs out of retirement and asking another if she would postpone her retirement during a COVID-19 surge.

At the intersection of surgery and infection prevention resides a sometimes-neglected opportunity to further minimize infection risk by modernizing choices and innovation.

The International Association of Healthcare Central Service Materiel Management is the premier association for professionals in health care central service/materiel management. IAHCSMM provides structural educational opportunities, professional development and a forum for information exchange to more than 38,000 members and certificants. For more information, visit http://www.iahcsmm.org.
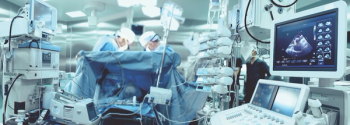
Many infection preventionists trust that everyone working in the operating room knows what they are doing and many times shy away from going into the OR. That's a mistake.
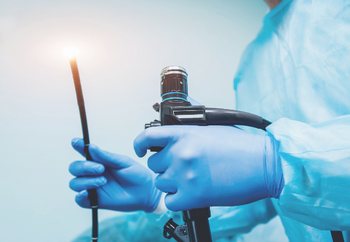
The FDA wants providers to know about “contamination issues associated with reprocessing urological endoscopes, including cystoscopes, ureteroscopes and cystourethroscopes—devices used to view and access the urinary tract.”
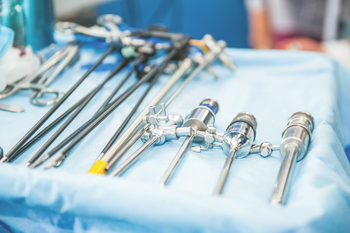
Tanya Lewis, CRCST: “I just think that infection preventionists and sterile processors should always work as a team. It should always be a team effort. It’s not them or us. It’s not sterile processing. It’s not infection prevention, but it’s us as a team. And that’s the way we’re going to keep our patients safe.”

Stethoscope diaphragms are contaminated with the same pathogens as the hands and they are capable of transmitting pathogens from patient to patient. The CDC should readdress its published guidelines.

Too often the tracking of the use and disinfection is done with pen and paper. That's what leads to problems, says Michael Cousin.
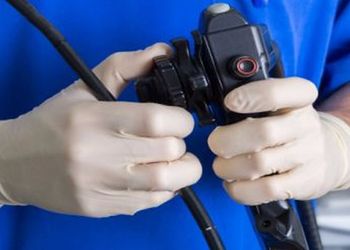
Linda Spaulding RN, BC, CIC, CHEC, CHOP: “Infection preventionists need to learn how to clean an endoscope, or at least observe the cleaning…. Infection preventionists need to make rounds, they need to talk to the person processing.”
Though tough months lie ahead for infection preventionists and other healthcare professionals, hope remains that at some point in 2021 things will begin to settle down. In the end, it comes down to a simple formula: We win, COVID-19 loses.

W. Frank Peacock, MD, FACEP, FACC, FESC: “When I intubate somebody, I need to know where the tube is, and I need to know now—like within 10 seconds. You can’t tell with anything else. Nothing is as fast as the stethoscope. I can get an X-ray, but I’ve got to wait for the X-ray while you hold your breath.”